
A1CNOW® Test Systems
Easy and Efficient
- Displays results in 5 minutes
- Requires only a small (5 µL) fingerstick blood sample
- Requires no maintenance
- Can be stored at room temperature
- Is small, compact, and battery-powered
- Is affordable and easy-to-use
Certified
- NGSP-certified
- IFCC-traceable
- CE-marked for self-test use
- CLIA-waived

About A1C
The term HbA1c refers to glycated hemoglobin. It develops when hemoglobin, a protein within red blood cells that carries oxygen throughout your body, joins with glucose in the blood, becoming ‘glycated.’ By measuring glycated hemoglobin (HbA1c), clinicians are able to get an overall picture of what our average blood sugar levels have been over a period of 2 to 3 months. For people with diabetes this is important as the higher the HbA1c, the greater the risk of developing diabetes-related complications. HbA1c is also referred to as hemoglobin A1C or simply A1C.
The answer is that people with diabetes should talk to their physician about how often they should test their A1C.
The Mayo Clinic recommends on its website that A1C testing varies dependent on the type of diabetes the person has, the treatment plan and how well the person with diabetes is managing their blood sugar. For example, the A1C test may be recommended…
- Twice a year if the patient has type 2 diabetes, doesn’t use insulin, and the blood sugar level is consistently within target range
- Three to four times a year if the patient has type 1 diabetes
- Four times a year if the patient has type 2 diabetes, uses insulin to manage diabetes, or has trouble keeping the blood sugar level within target range
People with diabetes may need more frequent A1C tests if the physician changes the diabetes treatment plan or the patient begins taking a new diabetes medication.
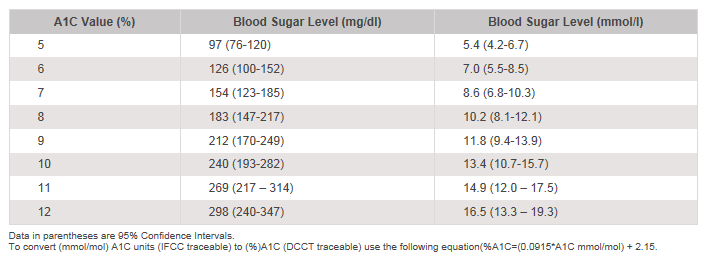
- Nathan, DM, Kuenen, Borg, R, Zheng, H, Schoenfeld, D, Heine, RJ. “Translating the A1C Assay Into Estimated Average Glucose Values” Diabetes Care Volume 32 (8), August 2008.
- NGSP Website: http://www.ngsp.org/ifcc.asp
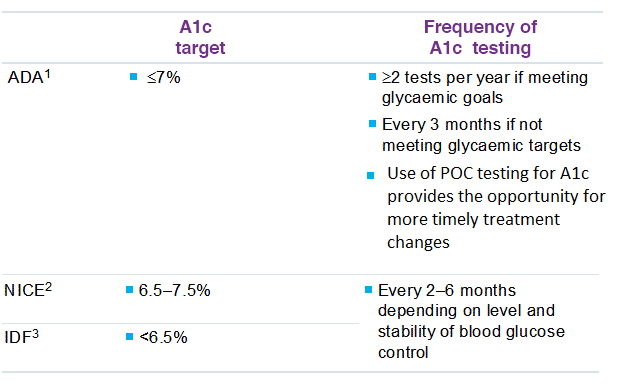
The American Diabetes Association (ADA) recommends A1C testing to determine a patient’s average blood glucose control. For patients whose therapy has changed or who are not meeting glycemic goals, the A1C test should be performed quarterly. The A1C test should be performed at least two times a year in patients who are meeting treatment goals and who have stable glycemic control. Guidelines about A1C testing by the American Diabetes Association, International Diabetes Federation, and National Institute for Health and Clinical Excellence are summarized in the table below.
Guidelines for A1C testing in patients with diabetes
The goal of therapy is to achieve an A1C as close to the non-diabetic range as possible without severe hypoglycemia.
References
- ADA Diabetes Care 2014; 37 (Suppl 1):S14-80
- https://www.nice.org.uk/guidance/qs6
- International Diabetes Federation. Recommendations For Managing Type 2 Diabetes In Primary Care, 2017. www.idf.org/managing-type2-diabetes
Less stringent treatment goals than those noted in the chart above may be appropriate for patients with a history of severe hypoglycemia, patients with limited life expectancies, very young children or older adults, and individuals with comorbid conditions.1
References
- American Diabetes Association. Executive Summary: Standards of Medical Care in Diabetes-2009. Diabetes Care, 32 (S1) 2009, pp. S6-S7.
Maintaining A1C levels as close as possible to normal limits has been shown to lower the risk of micro- and macrovascular complications. Despite the advent of novel therapies, historically, diabetes management has failed to achieve and maintain glycemic targets. Therefore, the EASD and ADA released joint consensus guidelines to support HCPs by providing treatment algorithms for effective diabetes management.
The Guidelines and Treatment Algorithm emphasize[1,2]
- Importance of achieving and maintaining normal glycemic goals
- Initial therapy with lifestyle modifications and metformin
- Rapid addition of medication and transition to new regimens when glycemic goals are not achieved or sustained
- Early addition of insulin therapy in patients who do not meet glycemic goals.
- The guidelines acknowledge the important role of SMBG, particularly to help achieve glycemic goals with the latter two treatment regimens
Diagnosis
Lifestyle intervention and metformin
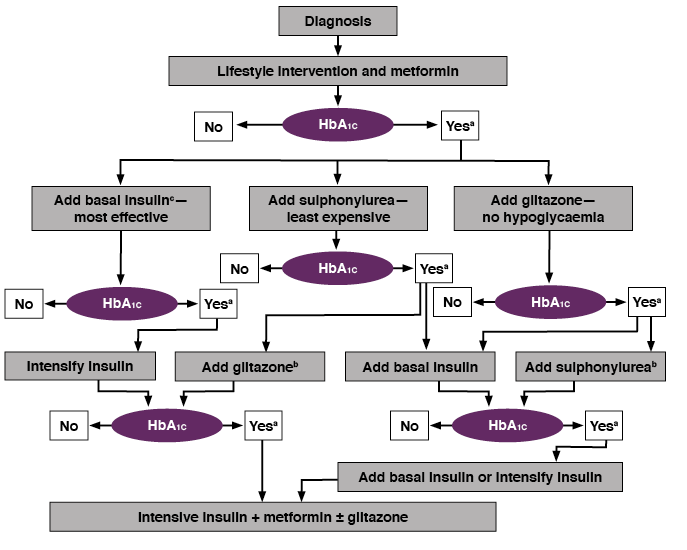
a. Check HbA1c every month until HbA1c is <7%, and then at least every 6 months.
b. Although three oral agents can be used, initiation and intensification of insulin therapy is preferred based on effectiveness and expense.
c. See algorithm for initiation and adjustment of insulin.
Nathan D, et al. Diabetes Care 2006;29:1963–72. Reproduced with permission.
References
- Nathan et. al. “Management of Hyperglycemia in Type 2 Diabetes: A Consensus Algorithm for Initiation and Adjustment to Therapy”, Diabetes Care 2006, 29 (8), pp. 1963-1973.
- Inzucchi Ctal “Management of Hyperglycemia in Type 2 Diabetes: A Patient Centered Approach”, Diabetes Care 2012, 35: 1364-1379
The Diabetes Control and Complications Trial (DCCT) was the pivotal trial that provided the link between A1C levels and the risk of diabetes-associated complications. The results of the DCCT shown below are considered definitive for patients with type 1 diabetes. Relative risk increased with A1C for retinopathy, nephropathy, and microalbuminuria, and the risk of retinopathy and nephropathy accelerated at the highest levels of A1C. In this study, improved glycemic control following intensive diabetes therapy delayed the onset and slowed the progression of diabetic retinopathy, nephropathy and neuropathy in patients with type 1 diabetes.1
DCCT A1C levels and the risk of complications in type 1 diabetes
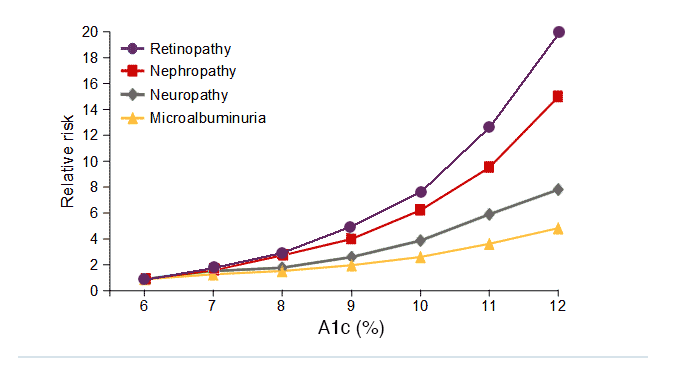
Adapted from DCCT. Diabetes 1995;44:968-43.
The United Kingdom Prospective Diabetes Study (UKPDS) was a large-scale trial that investigated the effect of intensive blood glucose control versus conventional treatment in patients with type 2 diabetes, with a median follow-up of 10 years. This observational analysis of data from the UKPDS demonstrated a direct relationship between the risk of diabetic complications and glycemia over time. Each 1% absolute reduction in mean A1C levels was associated with a 37% decrease in the risk of microvascular complications and a 21% reduction in the risk of any diabetes-related complication or death.
Therefore, any improvement in A1C levels is likely to reduce the risk of diabetic complications. 2
Lowering A1C levels reduces the risk of diabetes complications in people with type 2 diabetes
UKPDS: 21% risk reduction per 1% absolute decrease in A1C levels (p<0.0001)
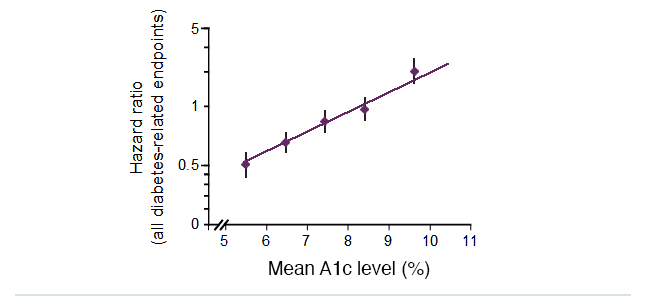
Slide from: http://www.dtu.ox.ac.uk/generic/slides.php
References
- DCCT. Diabetes 1995;44:968–83.
- UKPDS 35. BMJ 2000;321:405–12.
A1CNow+ is fast, easy, and accurate. It provides A1C results in 5 minutes which are 99% laboratory accurate*. These A1C tests are for use in a doctor’s office. Providing real-time results outside of the laboratory, A1CNow+ is a hand-held, portable monitor which allows A1C testing in every exam room. Real-time A1C results allow for timely decisions on therapy changes when needed. The product requires no maintenance. Office staff no longer have to prepare and refrigerate lab samples. Obtaining A1C results during the patient office visit eliminates follow-up phone calls to discuss results and possible therapeutic changes. Our A1CNow® System is CLIA waived and certified by the National Glycohemoglobin Standardization Program (NGSP).
* Study results with healthcare professionals showed that the accuracy of A1CNow+ with fingerstick samples was, on average, 99%. This means that, on average, a true 7.0% A1C could read approximately 6.9% A1C. An individual A1CNow+ result may differ by as much as -1.0% A1C to +0.8% A1C from the true result. This represents the 95% confidence limits of a Bland-Altman plot.
The single-use test provides quantitative results from a single drop of blood. Lance the finger for an adequate drop of blood. Touch the blood drop with the tip of the blood collector. Insert the blood collector into the open end of the sampler body and shake. Insert the cartridge into the A1CNow+ monitor. Deliver the sampler body into the cartridge. Your result will display after 5 minutes. Results are 99% laboratory accurate*.
*Study results with healthcare professionals showed that the accuracy of A1CNow+ with fingerstick samples was, on average, 99%. This means that, on average, a true 7.0% A1C could read approximately 6.9% A1C. An individual A1CNow+ result may differ by as much as -1.0% A1C to +0.8% A1C from the true result. This represents the 95% confidence limits of a Bland-Altman plot.
The NGSP standardizes glycated hemoglobin test results so that clinical laboratory results are comparable to those reported in the Diabetes Control and Complications Trial (DCCT) where relationships to mean blood glucose and risk for vascular complications have been established. A key component of the program is the Reference Laboratory Network. The network interacts with manufacturers of glycohemoglobin methods to assist them first in standardizing their methods and then in providing comparison data for certification of traceability to the DCCT.1
References
Clinical Laboratory Improvements Amendment (CLIA) is an act of Congress that established quality standards for all laboratory testing to ensure the accuracy, reliability, and timeliness of patient test results regardless of where the test was performed.1
A CLIA-waived test has been reviewed by the Food & Drug Administration (FDA) and Centers for Medicare and Medicaid Services (CMS). It is defined as simple laboratory examinations and procedures that are cleared by the FDA for home use; employ methodologies that are so simple and accurate as to render the likelihood of erroneous results negligible; or pose no reasonable risk of harm to the patient if the test is performed incorrectly.2
References
- http://www.cms.hhs.gov/clia/
- http://www.fda.gov/medicaldevices/deviceregulationandguidance/ivdregulatoryassistance/ucm393233.htm





