Lab Quality Results at the Point of CarE
References 1. Summary of 2017 Evaluations Comparing the A1CNow+ System to Clinical Lab Analyzers. Data on file. 2. http://www.ngsp.org/bground.asp
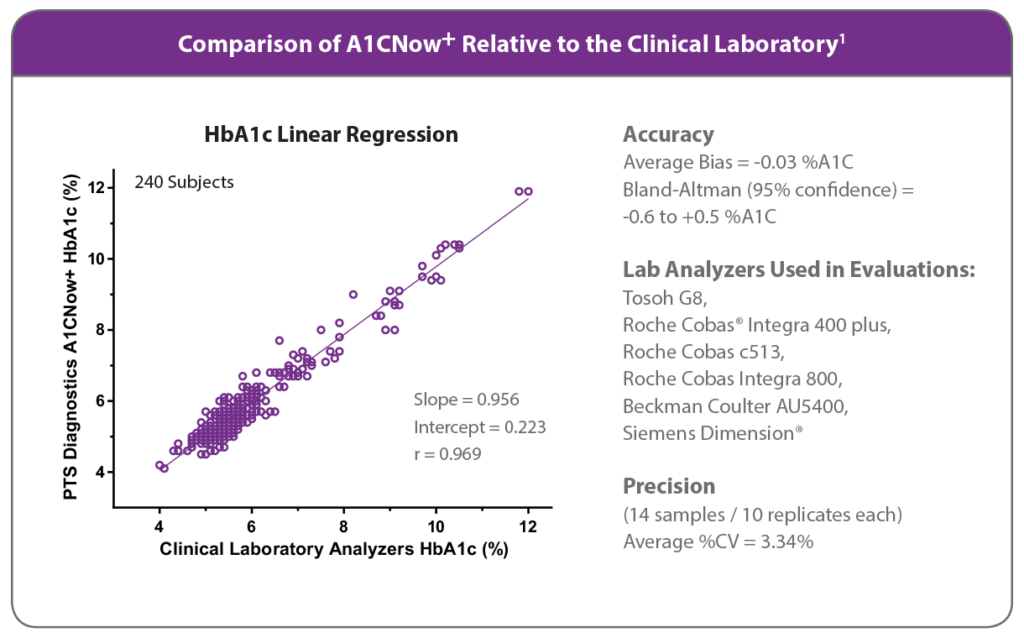
About A1CNow Accuracy and Performance
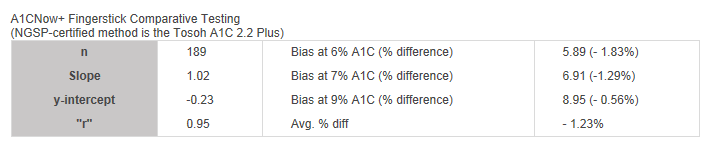
A1CNow+ Fingerstick Comparative Testing
(NGSP-certified method is the Tosoh A1C 2.2 Plus)
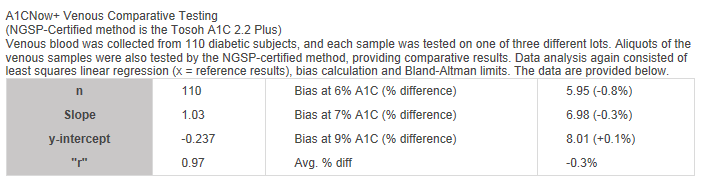
A1CNow+ Venous Comparative Testing
(NGSP-Certified method is the Tosoh A1C 2.2 Plus)
Venous blood was collected from 110 diabetic subjects, and each sample was tested on one of three different lots. Aliquots of the venous samples were also tested by the NGSP-certified method, providing comparative results. Data analysis again consisted of least squares linear regression (x = reference results), bias calculation, and Bland-Altman limits. The data are provided below.
References
- Professional product insert P/N 90821B.
Precision is the quality of being repeatable in amount or performance. Precision of a test is observed when measurement of a sample has nearly the same value each time it is measured. Precision testing was done under a specialized protocol. Following this protocol, two whole blood samples, one of approximately 6.0% A1C (low), and one of approximately 9.0% A1C (high), were tested over 20 days and four runs per day, for a total of 80 assays per level. The overall imprecision (including within-day and between-day) was 3.00% CV at the low level and 4.02% CV at the high level.1
References
- Professional product insert P/N 90821B.
The purpose of the NGSP is to standardize Hemoglobin A1C
test results to those of the Diabetes Control and Complications Trial (DCCT)
and United Kingdom Prospective Diabetes Study (UKPDS) which established the
direct relationships between HbA1c levels and outcome risks in patients with
diabetes.
Source: http://www.ngsp.org/index.asp
The NGSP offers three types of certification: manufacturer
method certification, Level I Laboratory Certification, and Level II Laboratory
Certification. The certification process includes the exchange of 40 patient
samples and an assessment of agreement analysis. A certificate of traceability
to the Diabetes Control and Complications Trial (DCCT) Reference Method is
issued to any manufacturer or laboratory that successfully completes the
certification process. The certificate is effective for 1 year and is specific
to the reagents and instrumentation used during certification.
|
Summary of Certification Criteria
|
Source: http://www.ngsp.org/critsumm.asp
A1CNow test systems are IFCC-traceable. The relationship between the National Glycohemoglobin Standardization Program (NGSP) A1C calibration (expressed as % A1C) and the International Federation of Clinical Chemistry (IFCC) network calibration (expressed as mmol/mol) has been shown to be stable and is given by the master equation: (NGSP = [0.09148 * IFCC] + 2.152). For further details and additional references, see the NGSP website at http://www.ngsp.org/.Since A1CNow test systems are NGSP-certified results can be converted to IFCC units with the master equation.
Demonstrating IFCC traceability is a formal process during which A1C blood samples are assigned values by the IFCC network. Manufacturers use these samples to verify alignment of their assay with the IFCC network.
References
National Glycohemoglobin Standardization Program webpage, http://www.ngsp.org
There will always be differences between multiple test results due to normal variation, time between tests, and differences between methods. In fact, duplicate test results from the same patient will rarely match even when the test is performed using the same method at the same time. Differences can sometimes appear greater because one result could be slightly higher and the other could be slightly lower than the reference (true) value. Positive and negative biases are often present in all of the most frequently used A1C testing methods. Multiple A1C methods should not be used to evaluate a
single patient or the performance of an A1C testing device because large and sometimes unacceptable inter-method differences are possible when multiple A1C testing devices are used for an individual patient.1
References
1 Holmes, E.W. et. al. (2008). Analytic Bias among Certified Methods for the Measurement of Hemoglobin A1c. American Journal of Clinical Pathology, 129, 540-547.
While in most people Hemoglobin A accounts for 95 to 98% of their hemoglobin, some individuals have genetic variations (such as Hemoglobin S or Hemoglobin F) that could affect the accuracy of a HbA1c test.
For more information, please refer to Sickle Cell Trait and Other Hemoglobinopathies and Diabetes: Important Information for Physicians (National Diabetes Information Clearinghouse)

