A1CNow+ System Correlation Study Guidelines
A1CNow®+ System Correlation Study Guidelines
Purpose:
These guidelines are suggestions for healthcare professionals who wish to evaluate the laboratory performance of the A1CNow+ system in their own clinical setting. The purpose of a correlation study (also known as a Method Comparison study) is to establish the relationship of an existing method to a new method. A minimum of 20 blood samples are suggested to have a valid number of data points for regression analysis. A sample size of 30 or more is highly recommended to increase the likelihood of attaining robust statistical results.
Subject Informed Consent:
When conducting an evaluation of the system, it will be necessary to follow your Institutional Review Board (Ethics Committee) policies concerning the protocol and an informed consent for collecting blood samples. Consult with your local institutional authorities. Study supplies provided by PTS Diagnostics (PTS) are strictly for the purpose of the correlation study to evaluate product performance. PTS does not assume any clinical investigator responsibilities. Results obtained from the correlation study are for product performance measures only and are not to be used for the purposes of diagnosis or treatment.
References:
1 EP15-A2, User Verification of Performance for Precision and Trueness, Approved Guideline, 2nd Edition; Clinical and Laboratory Standards Institute, 2006.
2 National Glycohemoglobin Standardization Program (NGSP) Manufacturer Information Packet; University of Missouri, NGSP website (www.ngsp.org).
3 Laboratory Statistics, Fun and Easy, A Practical Approach to Method Validation, David G. Rhodes, 1996.
Materials Required:
1. A1CNow+ professional-use product samples
2. Fingerstick and/or fresh whole blood (heparin) patient samples in the ranges and quantities listed below are recommended for accuracy testing:
Table: Suggested Distribution of Samples
HbA1c Sample Range if n=20 if n=30
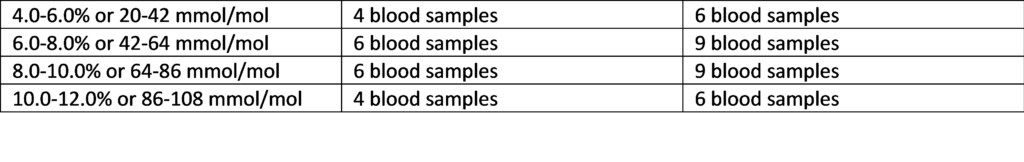
Samples with known hemoglobin variants (e.g., HbS or HbC), known conditions that affect red blood cell life span (e.g., anemia, pregnancy), or with known high titers of Rheumatoid Factor should not be included in the evaluation. Refer to the product insert for additional information.
LIT 001688 Rev.1 04/17
Procedure:
Ensure the operator is familiar with all procedural steps outlined in the product insert and procedure guide before beginning this evaluation. All A1CNow+ product must be at room temperature and well-mixed before testing.
PTS highly recommends performing comparison testing against a National Glycohemoglobin Standardization Program (NGSP) certified method as a gold standard reference or IFCC, depending on requirements within your country. Testing should include at least 20 patient blood samples (preferably 30) in the ranges stated in the “Materials Required” section on page one of this protocol. Record each result on the Glycohemoglobin Data Collection Form included in this packet.
Correlation Study Steps:
1. Affix a label to each blood collection tube and check off the corresponding number on the Glycohemoglobin Data Collection Form.
2. Collect patient blood samples. Accuracy testing is best performed using fingerstick samples for A1CNow+ systems. Otherwise, samples should be fresh, whole blood preserved with Sodium or Lithium Heparin (green top tubes).1 If necessary, blood samples should be stored in the refrigerator (2-8ºC) and brought to room temperature and well-mixed before use. Blood samples should be run on the same day simultaneously when using multiple instruments at the site to avoid introducing additional variation.
3. Perform testing on a comparative method (in-office device or reference lab). Record results on your Glycohemoglobin Data Collection Form. Once completed, make a copy of the Glycohemoglobin Data Collection Form for your records.
4. Data can be calculated by PTS Diagnostics, if desired.
5. Send blood samples to an NGSP or IFCC certified lab.
a. Refrigerate all blood samples at 2-8ºC until ready for shipping. Ensure collection tube caps are secure.
b. Once sample collection is completed (or within 7 days of sample collection), place all samples into the specimen transport bag along with some absorbent material. Place the ice pack in the bottom of the cooler. Wrap the specimen bag with bubble wrap and place in cooler. The samples should not touch the ice pack. Place lid on the cooler and place cooler inside the shipping box.
c. Complete the Shipment Packing List and place on top of cooler. Securely close and tape the shipping box and affix pre-addressed FedEx label.
Within the United States:
d. Drop the box off at your local FedEx location or call FedEx to schedule a pick up (800-463-3339). NOTE: Samples must be shipped Monday through Thursday only; the laboratory is not open to receive samples on the weekends.
e. Ship to:
PTS Diagnostics
510 Oakmead Parkway
Sunnyvale, California 94085
(408) 524-2255
Attn: Charles Xie
Outside the United States:
f. NGSP or IFCC certified labs within your home country should be utilized.
http://www.ngsp.org/docs/labs.pdf
A Note on Reference Range:
Reference ranges apply to expected values in a normal, healthy population. Since HbA1c results are applicable to diabetic populations, conventional reference ranges are not relevant. However, for informational purposes, the A1CNow+ system was tested with 118 non-diabetic individuals across three US sites. The mean HbA1c result was 5.2% (33 mmol/mol) with a ± 0.71, 1 SD and the 95% confidence limits were 3.9% (19 mmol/mol) to 6.5% (48 mmol/mol). If your laboratory wishes to establish its own reference range, it is necessary to test approximately 120 non-diabetic individuals.2,3 A more useful clinical practice tool would be to set ranges for treatment goals. Visit www.A1CNow.com for more information.
1 NOTE: The use of heparin/green top tubes will not affect testing results, even if your reference lab typically uses EDTA/purple top tubes for A1C testing.
2 NCCLS C28-A2, How to Define and Determine Reference Intervals in the Clinical Laboratory; Approved
Guideline-Second Edition (2000).
3 Tietz Textbook of Clinical Chemistry, Third Edition. W.B. Saunders Company, 1999

